Connected devices play a crucial role in modernizing and improving the efficiency of clinical trials. These devices, often part of the broader concept of the Internet of Things (IoT), can collect real-time data, enhance patient monitoring, and streamline the overall trial process.
Here are several ways in which connected devices contribute to effective clinical trials:
Remote Patient Monitoring (RPM):
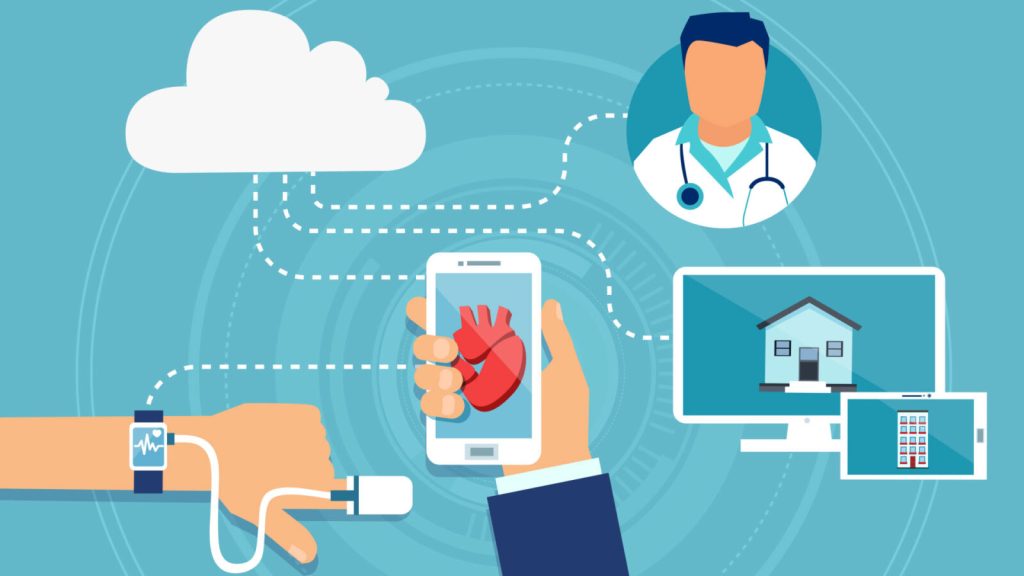
Connected medical devices, such as glucometers, blood pressure monitors, and spirometers, enable real-time data collection. This data can be transmitted securely to healthcare providers, allowing for remote monitoring and immediate intervention if necessary. This continuous monitoring allows for a more comprehensive understanding of a patient’s health outside of traditional clinical settings.
Enhanced Patient Engagement:

Connected devices often link to mobile apps or online portals that facilitate communication between patients and researchers. This can include reminders for medication adherence, reporting symptoms, and accessing trial-related information and also patient-reported data on symptoms, quality of life, and other relevant information.. This information is valuable for understanding the patient experience during the trial.
Data Accuracy and Timeliness:

Connected devices can seamlessly integrate with EHR systems, reducing manual data entry errors and ensuring that trial data is accurate and up to date. Also connected device’s ability to collect real-time data from patients, rather than relying on periodic clinic visits, provides a more dynamic and accurate representation of a patient’s health status.
Improved Protocol Adherence:

These devices can be programmed to dispense medications at specific times and send reminders to patients. This helps improve medication adherence and ensures that the trial protocol is followed. For trials involving multiple study sites, connected devices with geolocation capabilities can help ensure that patients are where they need to be for study visits and assessments.
Efficient Data Analysis and Monitoring:

Connected devices generate large amounts of data. Utilizing data aggregation and analytics tools can help researchers quickly analyze and derive meaningful insights from this information, potentially accelerating the trial timeline. Connected devices can aid in the real-time monitoring of adverse events, allowing for swift intervention and ensuring participant safety.
The integration of connected medical devices in clinical trails holds the potential to revolutionize the way clinical research is conducted, making it more patient-centric, efficient, and data-driven.



You must be logged in to post a comment.